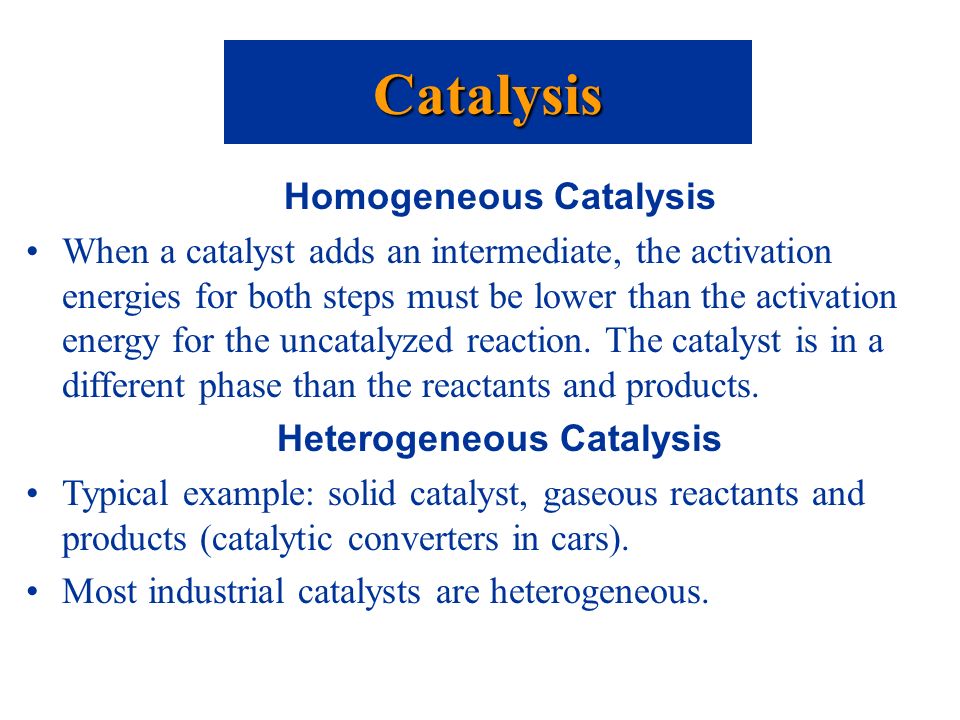
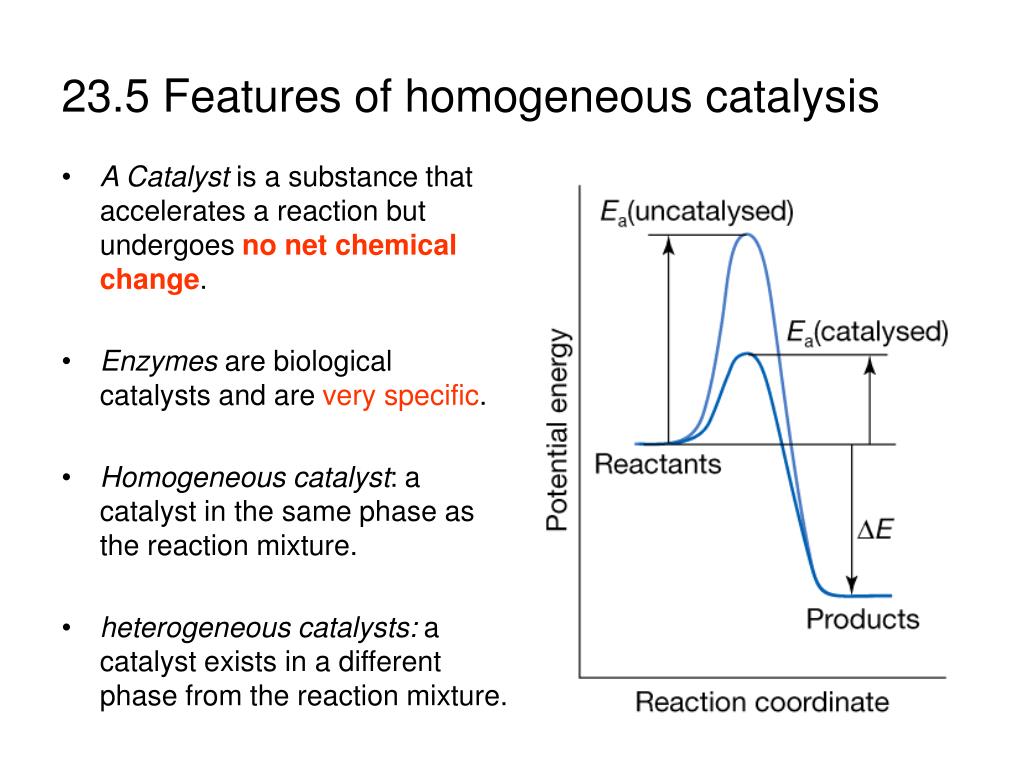
For one, heterogeneous catalysts can be separated from a reaction mixture in a straightforward manner, such as by filtration In this way, expensive catalysts can be easily and effectively recovered, which is an important consideration for industrial manufacturing processes Examples of Homogeneous Catalysts Acid catalysis, organometallicTypically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixture Catalysis Note the lowered activation energy of the catalyzed pathway Examples of Homogeneous Catalysts Acid catalysis, organometallic catalysis, and enzymatic catalysis are examples of homogeneous catalysis A catalyst which exists in a different phase from the reactants is known as a heterogeneous catalyst and the catalysis known as heterogeneous catalysis Generally, the Heterogenous catalysts are in a solid state, while the reactants are in the liquid or gaseous state

Which Of The Following Is An Example Of Heterogeneous Catalysis Reaction
How does a heterogeneous catalyst work
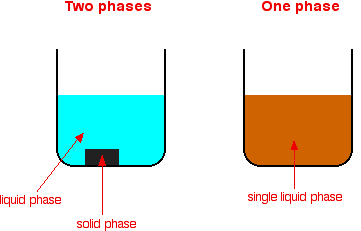
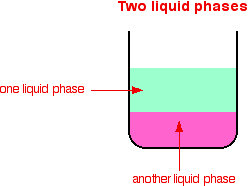
How does a heterogeneous catalyst work- Common examples for heterogeneous catalysts are metals, metal oxides, etc Figure 2 Reaction Mixture is in Liquid Phase whereas Catalyst is a Metal in Solid Phase The thermal stability of heterogeneous catalysts is very good compared to homogeneous catalysts These catalysts efficiently act in hightemperature conditions, around ◦C A catalyst is a compound used to help a reaction occur faster by lowering the activation energy There are two types of catalysts, homogeneous and heterogeneous A homogeneous catalyst is a



Heterogeneous Catalysis All About Drugs
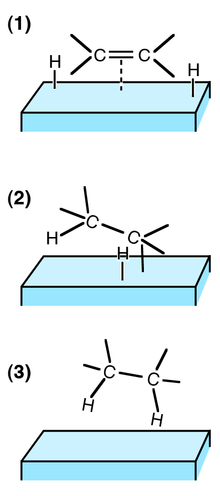
This is an example of heterogeneous catalysis It is heterogeneous catalysis because the catalyst is a solid and the reactants are gases Examples of homogeneous catalysis The reaction between persulphate ions and iodide ions This is a solution reaction that you may well only meet in the context of catalysis but it is a lovely example! Heterogeneous catalysts are used extensively in the petroleum industry One example is the combination of SiO 2 and Al 2 O 3 used to speed up cracking of longchain hydrocarbons into the smaller molecules needed for gasoline Another is the Pt catalyst used to reform hydrocarbon chains into aromatic ring structures Heterogeneous catalysis is the type of catalysis where the phase of the catalyst differs from the phase of the reactants This contrasts with homogeneous catalysis where the reactants and catalyst exist in the same phase Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (eg oil and water), or anywhere an interface is present Catalysts
One example of a heterogeneous catalyst is the catalytic converter in gasoline or dieselfueled cars Catalytic converters contain transition metal catalysts embedded on a solid phase support Then the mixture of the liquid substrate and solid catalyst is shaken or stirred in a hydrogen atmosphere However, then actual reaction takes place at the surface of the metal catalyst and is an example of heterogeneous or surface catalysis 11A dermoid cyst, for example, has a heterogeneous attenuation to CT For homogeneous, it is the antonym, indicating a system of identical components Heterogeneous refers to a system whose root is alien What are the two types of catalyst?
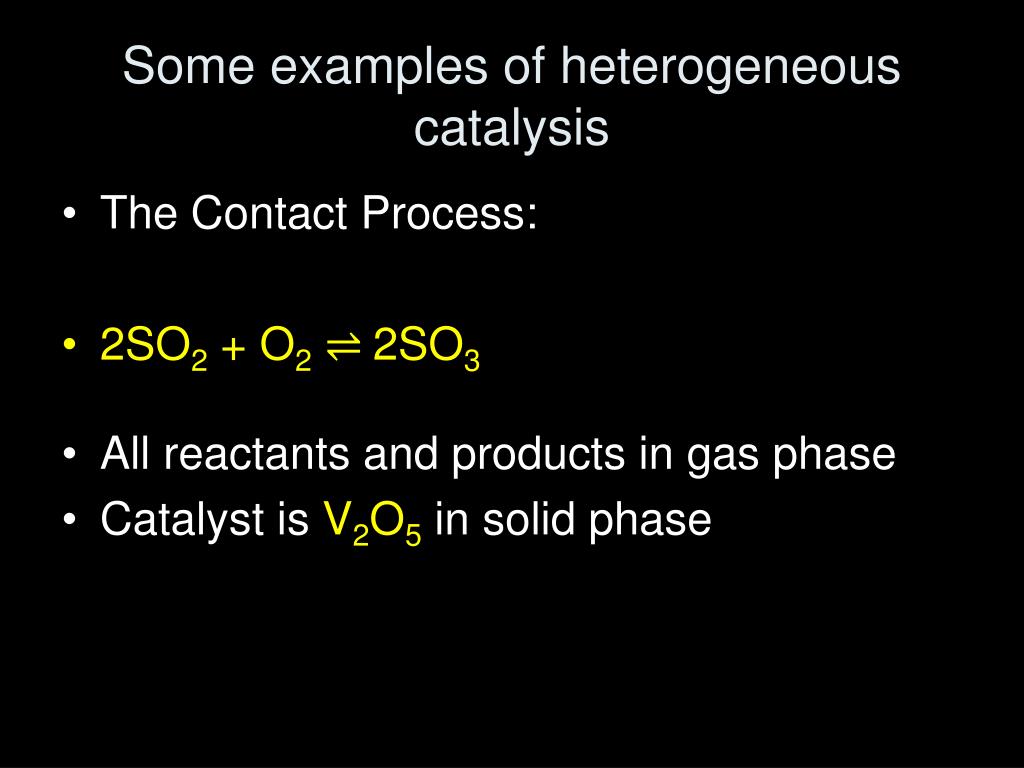
Heterogeneous catalysts are especially wellsuited for continuousprocess chemical reactions, in which material is provided, reacted, removed and replaced, continuously An example of such a heterogeneous catalyst process from the petroleum industry is the use of pelletized catalytic material in the socalled "moving bed" processIn chemistry, homogeneous catalysis is catalysis in a solution by a soluble catalyst Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solidgas, respectivelyIn which the reactants and catalyst are in a similar phase Example 2SO 2 (g) O 2 (g) —– No(g) → 2SO 3 (g) Heterogeneous catalysis Heterogeneous catalysts are catalytic compounds that are in a contradictory phase from that of the phase of the reaction combination Heterogeneous catalysis is found in the liquid phase, gas phase, and



Question 9 An Example Of A Heterogeneous Catalyst Is Chegg Com




Catalysis Heterogeneous Catalysis Britannica
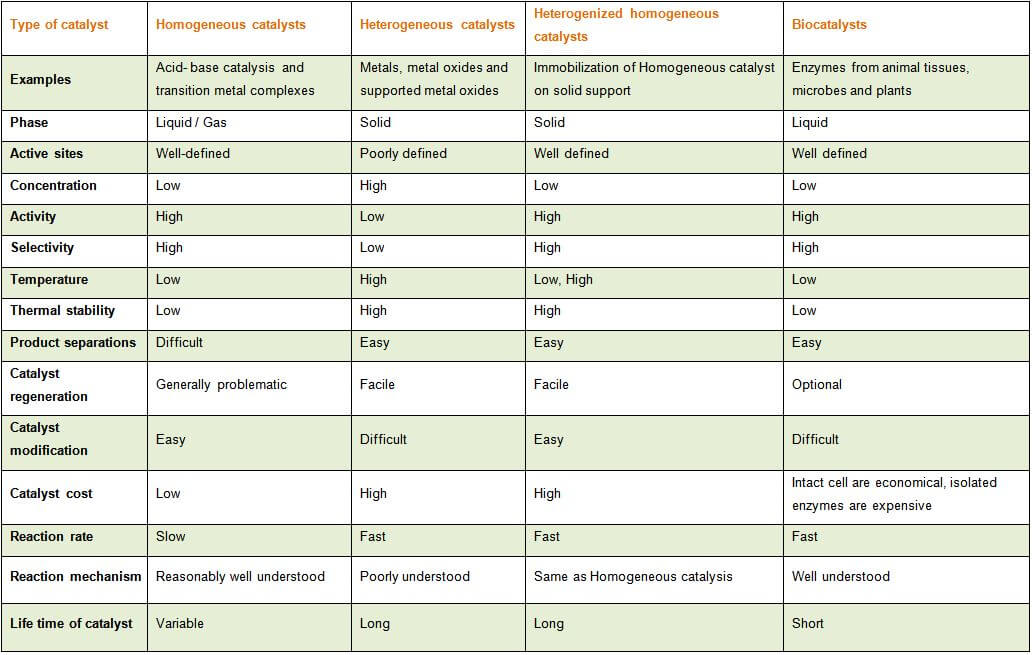
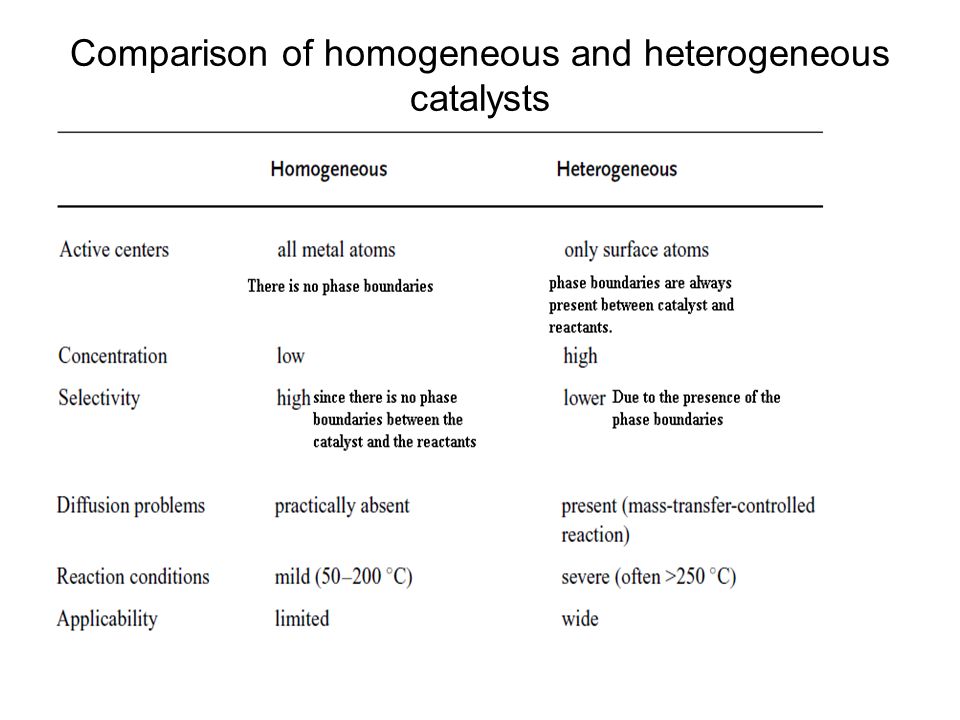
Catalysts in heterogeneous catalysis act in the same way as in homogeneous catalysis — by lowering the activation energy for the catalyzed reactions We will not discuss how catalysts actually achieve this, but we can briefly mention that most processes involve two types of solid catalysts Acid and metal catalysts (as per G F Froment, K BFor example, in the interaction of metaphosphoric acid with potassium persulfate, hydroiodic acid acts as a catalyst When it is added to the reactants, a yellow solution is formed As we approach the end of the process, the color gradually disappears In this case, iodine acts as an intermediate product, and the process occurs in two stagesIdentify the characteristics of heterogeneous and homogeneous catalysis • describe three examples of green heterogeneous catalysis Catalysis may reduce materials, waste and energy Heterogeneous are easily recycled and longlived but illdefined Homogeneous are more active and selective but expensive and hard to recover Asahi Cyclohexanol process




What Is Heterogeneous Catalysis Give An Example



An Important Example Of Heterogeneous Catalysis Chegg Com
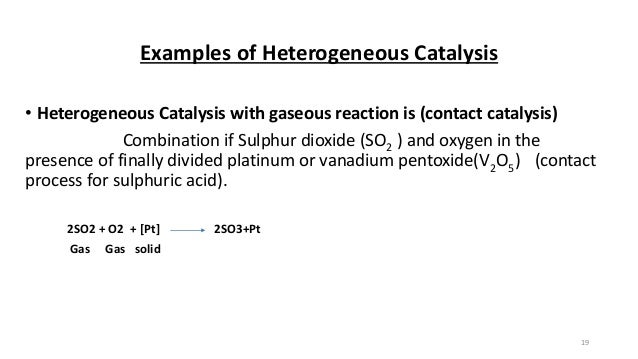
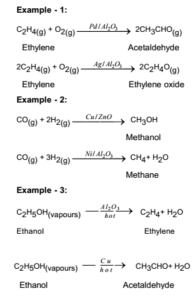
Homogeneous catalysis catalysis in which the reactants and catalyst are in same phaseIe same physical state Example 2 S O 2 (g) O 2 (g) → N O (g a s) 2 S O 3 (g) Heterogeneous catalysis catalysis in which the reactants and catalyst are in phases Ie different physical states Example 2 S O ( g) O ( g) → P t (s) 2 S O 3 (g)The most common examples of heterogeneous catalysis in industry involve the reactions of gases being passed over the surface of a solid, often a metal, a metal oxide or a zeolite (Table 1) Process Catalyst Equation Making ammonia Iron Making synthesis gasDifference Between Homogeneous Catalysis and Heterogeneous Catalysis Video Lecture from Surface Chemistry Chapter of Chemistry Class 11 for HSC, IIT JEE, CBS




A Selection Of Important Industrial Heterogeneous Catalysts And Their Download Table



Direct Arylation Using Heterogeneous Catalysts With Evidence Of Download Scientific Diagram
Heterogeneous catalysis This involves the use of a catalyst in a different phase from the reactants Typical examples involve a solidcatalyst with the reactants as either liquids or gases Note It is important that you remember the difference between theThis video provides a basic introduction into homogeneous and heterogeneous catalysts A Homogeneous catalyst exists in the same phase as the reactants andCatalysts are generally divided into two types, those that are in the same phase as the reactants (homogeneous catalysts) and those that belong to a different phase (heterogeneous catalysts) The first of the two reactions in this demonstration is an example of a homogeneous catalyst



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications




Heterogeneous Catalysis Wikipedia
For example, an SLP catalyst was developed for the facile hydrogenation of aromatics by impregnating silica or clay (attapulgite) with the solution of PtCl 2 (CH 3 CN) 2 in BF 3 ·H 2 O 14 Hydrogenation of arenes occurs rapidly even at 25 °C under 276 MPa H 2 and the colorless products can be separated from the catalyst by simple filtrationHeterogeneous catalysis Heterogeneous catalysis is a chemistry term which describes catalysis where the catalyst is in a different phase (ie solid, liquid and gas, but also oil and water) to the reactants Heterogeneous catalysts provide a surface for the chemical reaction to take place on Additional recommended knowledgeHETEROGENEOUS CATALYSIS Prof Shawky M Hassan Professor of Physical Chemistry Contents Chapter 2 Active sites of Heterogeneous Catalysis Chapter 3 Multiplet Theory 31 Geometric Factor 32 Energy Factor Chapter 4 Electronic Theory Chapter 5




Give Four Examples Of Heterogeneous Catalysis Brainly In




Which Reaction Is An Example Of A Heterogeneous Catalyst
Heterogeneous catalysis has traditionally been used for the largescale production of relatively simple molecules—for example, the Haber Bosch process to convert nitrogen and hydrogen into ammonia using Febased catalysts or catalytic cracking of long chain hydrocarbons using zeolites From Encyclopedia of Interfacial Chemistry, 18 For example, one may begin the catalytic reaction with the heterogeneous catalyst in a given oxidation state (eg, containing zerovalent metal particles following treatment of the catalyst in H 2 at elevated temperature); Examples include the HaberBosch process for the production of NH 3, catalytic cracking, and the hydrogenation of vegetable oils A heterogeneous catalyst provides a lower energy path via a sequence that involves adsorption of reactant molecules upon an active site in
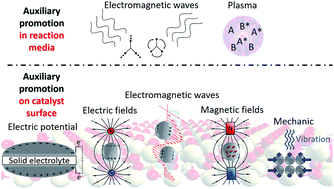



Promoting Heterogeneous Catalysis Beyond Catalyst Design Chemical Science Rsc Publishing
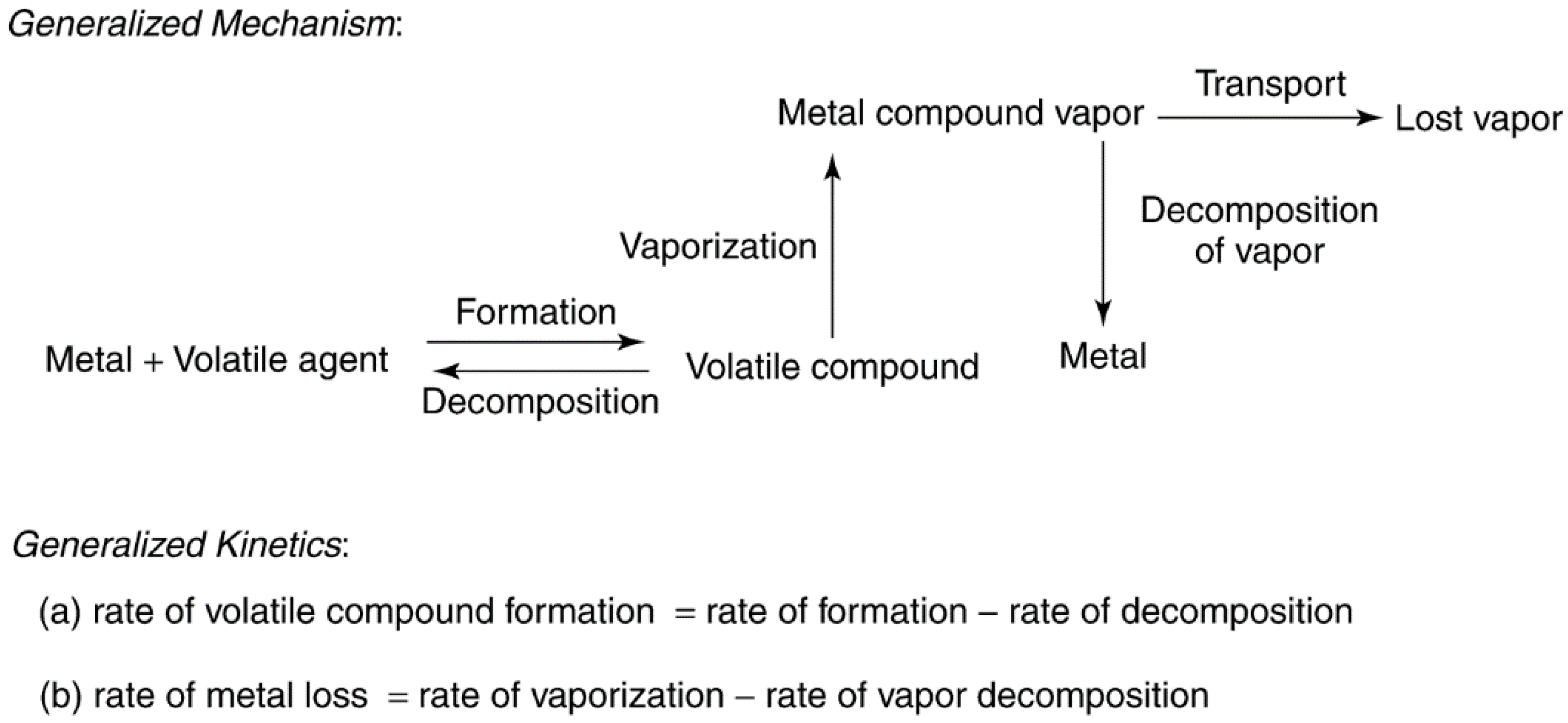



Catalysts Free Full Text Heterogeneous Catalyst Deactivation And Regeneration A Review Html
Compared to traditional heterogeneous catalysts such as oxides or supported metal particles The heterogeneity of the surface sites is, in fact, a common feature of the heterogeneous catalysts Property Homogeneous Heterogeneous Catalyst recovery difficult and expensive easy and cheap Thermal stability poor good2H2O2 (l) →Pt(s) 2H2O (l) O2(g)In this reaction, reactant and catalyst are in different phase, hence it is an example of heterogeneous catalysis Heterogeneous catalysis is a type of catalysis in which the catalyst occupies a different phase from the reactants and products Itmay refer to the physical phase solid, liquid or gas but also to immiscible fluidsDehydrogenation by Heterogeneous Catalysts Daniel E Resasco For example, under typical dehydrogenation conditions (550ºC, 1 atm) the equilibrium conversion types of catalysts a) Ptbased catalysts and b) chromiabased catalysts46 The main




Catalysts Transition Metals Secondary Science 4 All



A What Is A Heterogeneous Catalyst Explain Using Chegg Com
In recent years major progress has been made in the area of heterogeneous catalysis by metals Much has been learned about the nature of metal catalysts and of catalytic phenomena on metals Characteristic patterns of catalytic behavior among the metallic elements have been established for certain classes of reactions, and these patterns provide a first step toward a moreHowever, the nature of the surface can be changed dramatically upon interaction with strongly adsorbed species, such asAmong heterogeneous catalysts the supported ones are allimportant For their manufacture a material more or less inert to the reaction conditions is coated with the catalytically active substance in the form of aqueous solutions According to demand this support can be based on activated carbons, aluminas, silicas, calcium carbonate or




Heterogeneous Catalysis Definition Examples Diagrams




Which Of The Following Reactions Is Not An Example Of Chegg Com
Heterogeneous catalysts may be unsupported or supported Examples of unsupported catalysts include Raney nickel or cobalt These are made by chemically dissolving the aluminium out of nickelaluminium or cobaltaluminium alloys and they are used for the reduction of carbonyl functions, nitriles and oximes144 million tons of ammonia were produced in 16The chemical and energy industries rely heavily on heterogeneous catalysis For example, the Haber–Bosch process uses metalbased catalysts in the synthesis of ammonia, an important component in fertilizer;
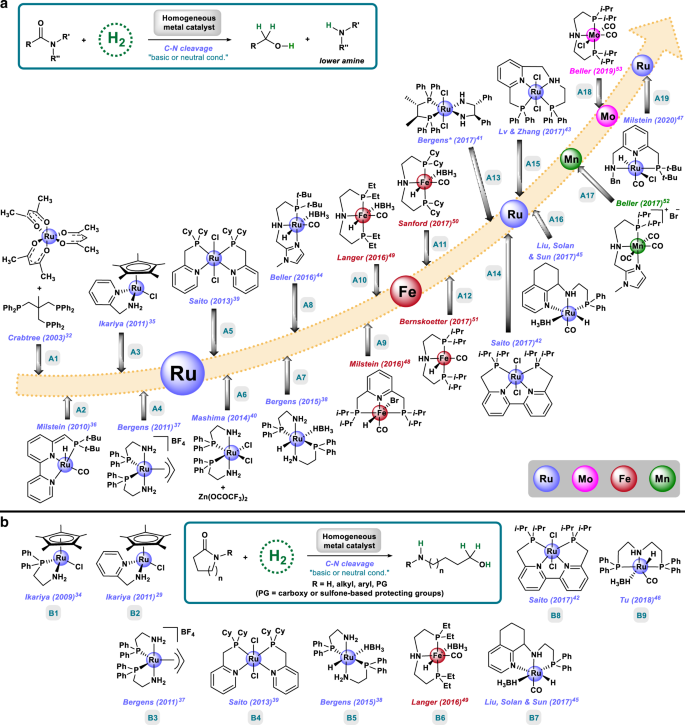



Homogeneous And Heterogeneous Catalytic Reduction Of Amides And Related Compounds Using Molecular Hydrogen Nature Communications
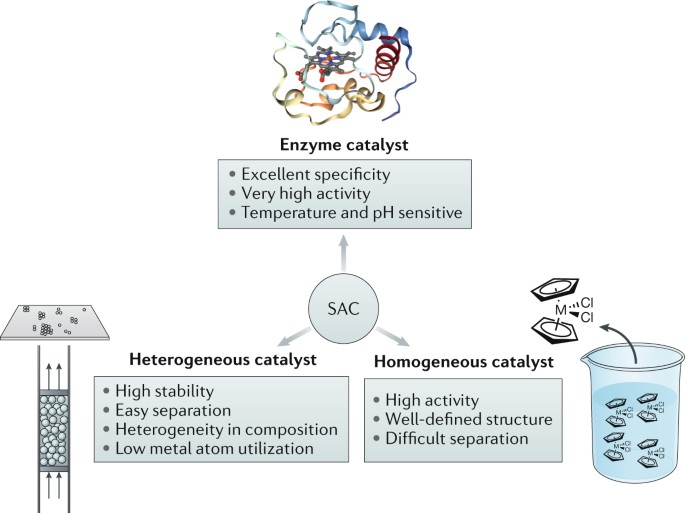



Heterogeneous Single Atom Catalysis Nature Reviews Chemistry
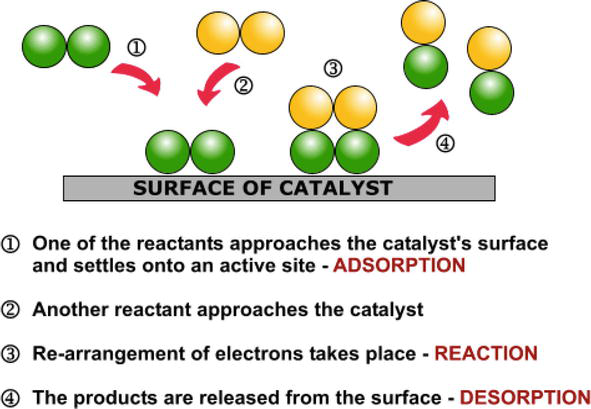
Homogeneous vs heterogeneous catalysis Dr habil Marko Hapke 5 5 Heterogeneous Catalysis Major industrial processes using heterogeneous catalysis Process Catalyst Reactants Products Application HaberBosch process Magnetite (Fe) H 2, N 2 NH 3 Fertiliser, explosives Methanol synthesis Cu/ZnO/Al 2O 3 CO, CO 2, HHeterogeneous catalysis The usual explanation for the mode of action of a heterogeneous catalyst is that of a template The catalyst provides a reactive surface that adsorbs one or both of the reactants molecules, weakening their bonds and increasing the rate of reaction between them Give four examples of heterogeneous catalysis Answer (i) Oxidation of sulphur dioxide to form sulphur trioxide In this reaction, Pt acts as a catalyst (ii) Formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron



Types Of Catalysis Homogeneous Catalysis Heterogeneous Catalysis Positive Catalysis Negative Catalysis Induced Catalysis Acid Base Catalysis



1 An Introduction To Types Of Catalysis Chemistry Libretexts
The catalysis in which the catalyst and the reactants are present in different phases is called heterogeneous catalysis A catalyst which exists in different phases from the reactants is known as heterogeneous catalyst Example Hydrogenation of unsaturated compounds catalysed by finely divided metals like Ni, Pd, PtCatalysis Catalysis Heterogeneous catalysis Many catalytic processes are known in which the catalyst and the reactants are not present in the same phase—that is, state of matter These are known as heterogeneous catalytic reactions They include reactions between gases or liquids or both at the surface of a solid catalyst Since the surface is the place at which the reactionUnfortunately, many industrial heterogeneous catalysts consist of active sites distributed on an amorphous surface such as silica, on nanoparticle surfaces, or in zeolites The complexity of these systems is such that the structure and reaction mechanism of




Heterogeneous Catalysis Wikipedia
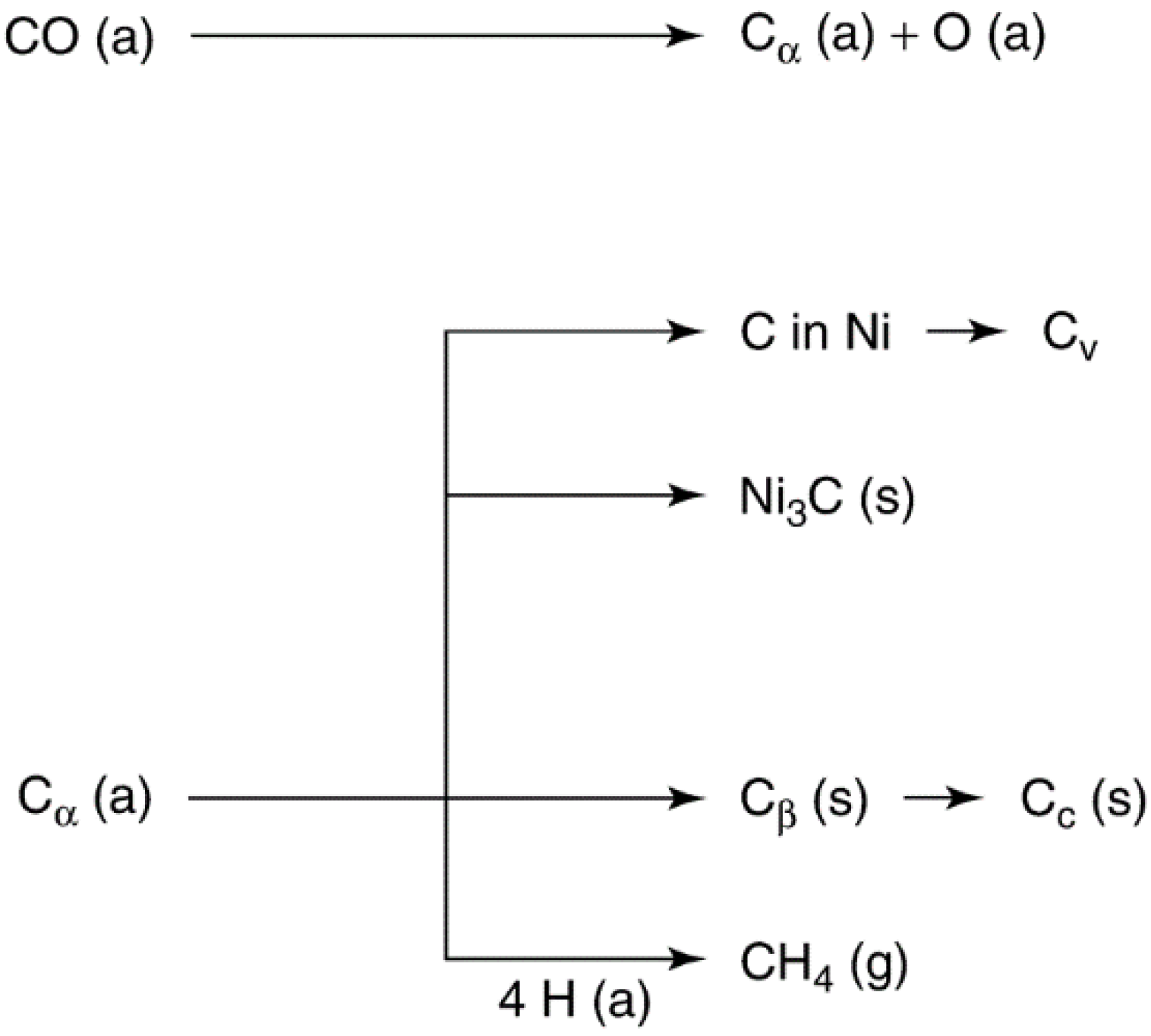



Catalysts Free Full Text Heterogeneous Catalyst Deactivation And Regeneration A Review Html
Catalyst Deactivation It is complex phenomenon which can be defined as loss of catalytic activity and / or selectivity Time scales for catalyst deactivation vary considerably;For example, in the case of cracking catalysts, catalyst mortality may be in the order of seconds While in ammonia synthesis the iron catalyst may last for 5–10 yearsHeterogeneous catalysts are extremely useful because they enable production of several commercially important products on a relatively large scale For example, oxides of iron placed on alumina (a chemical compound with the formula Al 2 O 3) are widely used as heterogeneous catalysts in the Haber process for the industrial production of ammonia



Catalysts For A Green Industry Feature Rsc Education




Types Of Catalysts Article Kinetics Khan Academy
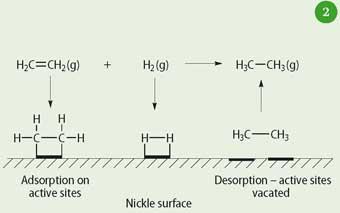
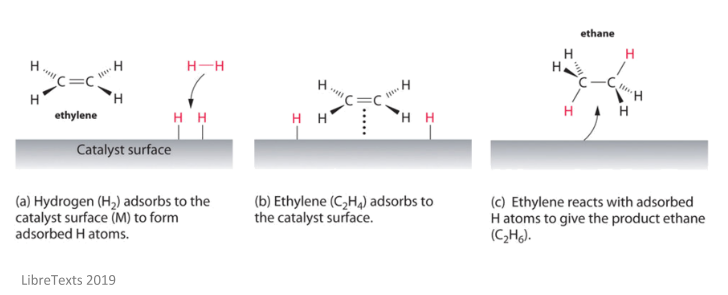
Examples of heterogeneous catalysisThe hydrogenation of a carboncarbon double bondThe simplest example of this is the reaction between ethene and hydrogen in the presence of a nickel catalyst In practice, this is a pointless reaction, because you are converting the extremely useful ethene into the relatively useless ethaneHomogeneous and heterogeneous catalysis Activity is the ability of the catalyst to accelerate a chemical reaction The degree can be as high as 100 times in certain reactions A catalytic cycle processes in which the reactant and catalyst undergo several transformations before making theHeterogeneous catalysts may be used as fine particles, powders, granules These catalysts may be deposited on the solid support (supported catalysts), or used in bulk form (unsupported catalysts) Supported catalyst play a pivotal role in the industrial revolution As heterogeneous catalysis is a surface phenomenon, the performance of catalysts
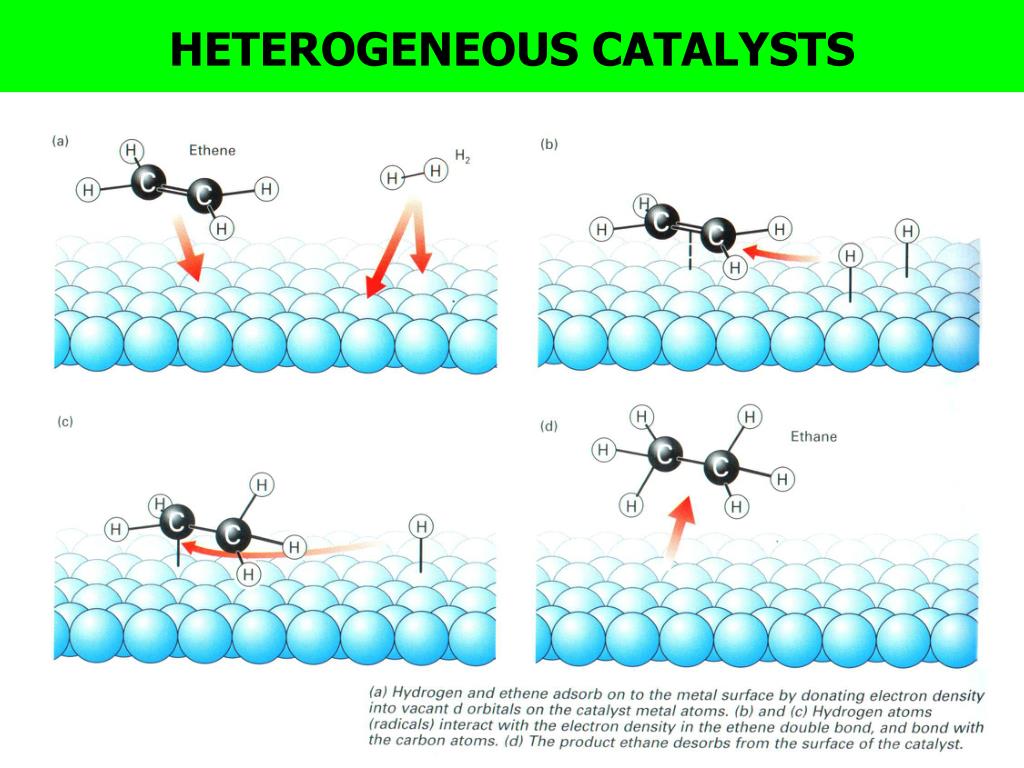



Ppt Starter 1 Definition Of Catalysts 2 Difference Between Homogeneous And Heterogeneous Catalyst Powerpoint Presentation Id



Catalyst Facts Summary Definition Chemistry Revision
Examples of Heterogeneous Catalysis and Catalysts – 1 In Haber's process of formation of ammonia, nitrogen and hydrogen are used in gaseous forms while catalyst iron




Explain The Difference Between A Homogeneous And Heterogeneous Catalyst Give An Example Of Each Youtube




Examples Of Possible Complexity Structures In Heterogeneous Catalysis Download Scientific Diagram




1 Catalytic Converter An Example Of Metallic Heterogeneous Catalyst Download Scientific Diagram




Revisiting Active Sites In Heterogeneous Catalysis Their Structure And Their Dynamic Behaviour Sciencedirect




A Selection Of Important Industrial Heterogeneous Catalysts And Their Download Table



Types Of Catalysis




Heterogeneous Homogeneous Catalysts Video Lesson Transcript Study Com



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora



1




Give Four Examples Of Heterogeneous Catalytic Reactions Youtube



Heterogeneous Catalytic Process For Wastewater Treatment Intechopen



1



Catalysis




Flow Fine Synthesis With Heterogeneous Catalysts Sciencedirect
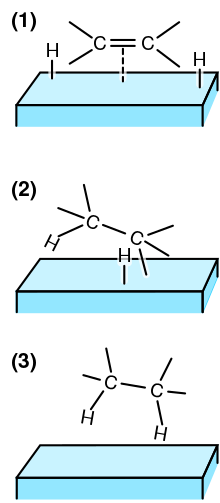



Heterogeneous Catalysis Wikipedia




Heterogeneous Catalysis Wikipedia




Heterogeneous Catalysis An Overview Sciencedirect Topics
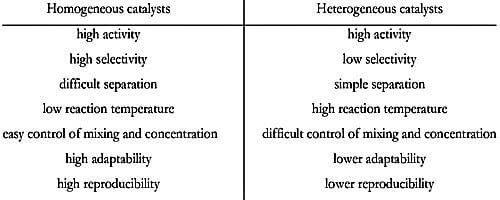



Differences Between Homogeneous Catalysis And Heterogeneous Catalysis Qs Study
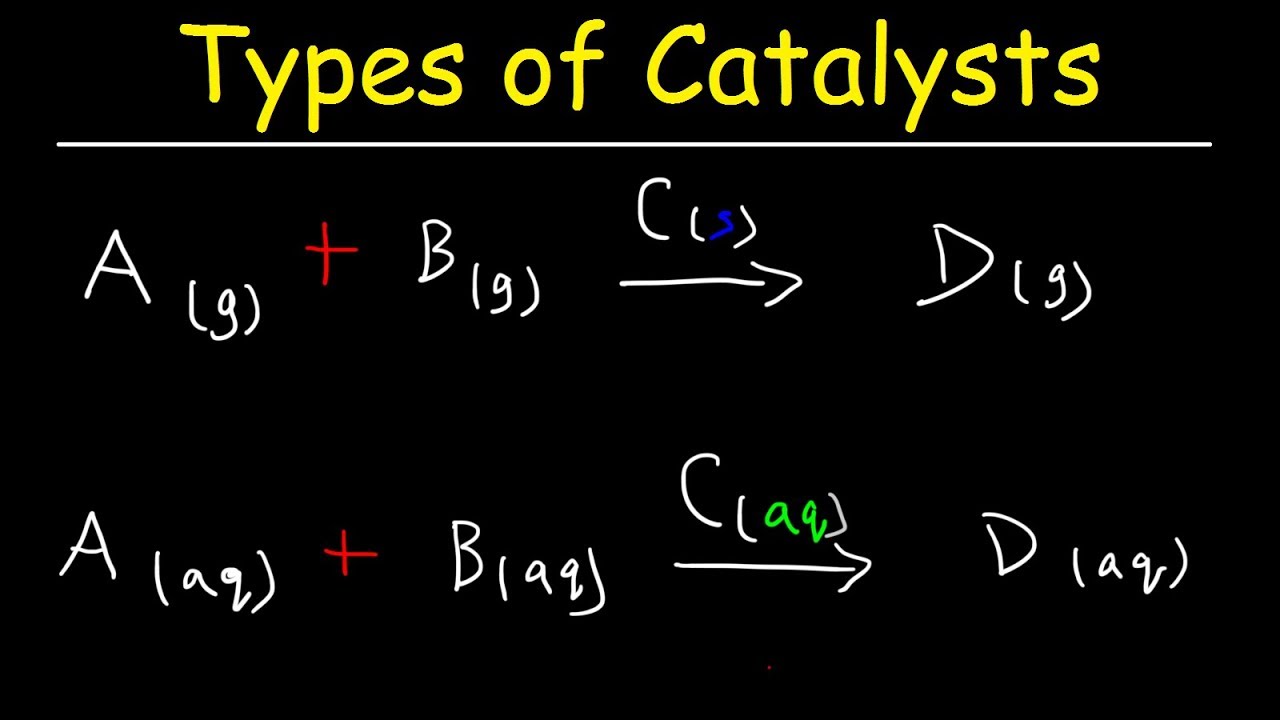



Homogeneous Vs Heterogeneous Catalysts Basic Introduction Youtube




Principles Of Heterogeneous Catalysis Dumesic Major Reference Works Wiley Online Library



1 An Introduction To Types Of Catalysis Chemistry Libretexts




Heterogeneous Catalysis Alchetron The Free Social Encyclopedia
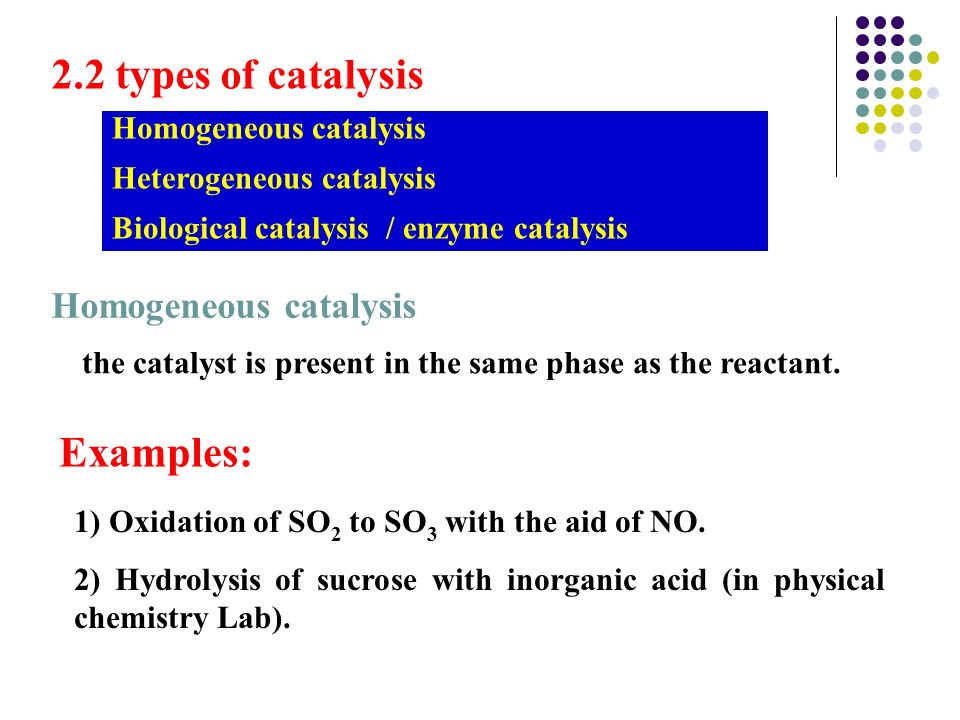



10 5 Catalytic Reaction Ppt Video Online Download




Which Of The Following Is An Example Of Heterogeneous Catalysis Reaction




Rate Processes Catalysts Rate Processes In Chemical Reactions Kinetics And Equilibrium Mcat Content




What Are Some Examples Of Homogeneous Catalysis Quora




Transition Metals Compounds Acting As Catalysis Catalytic Theory Practice Examples Of Homogeneous Ctalysts Heterogeneous Catalysis Gce As Ib A Level Inorganic Chemistry Revision Notes
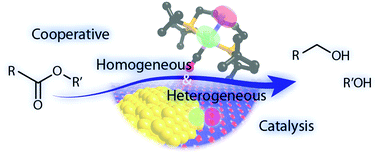



Heterogeneous And Homogeneous Catalysis For The Hydrogenation Of Carboxylic Acid Derivatives History Advances And Future Directions Chemical Society Reviews Rsc Publishing



Question 8 10 Points A What Is The Difference Chegg Com




Homogeneous Catalysis Wikipedia




Adsorption Theory Of Heterogeneous Catalysis Youtube




Catalysis Powerpoint Slides




Give Four Examples Of Heterogeneous Catalysis




Which Of The Following Is An Example Of Heterogeneous Chegg Com




Which Is An Example Of A Heterogeneous Catalyst Amylase Catalyt




Difference Between Homogeneous Catalysis And Heterogeneous Catalysis Surface Chemistry Youtube



Heterogeneous Catalysis All About Drugs
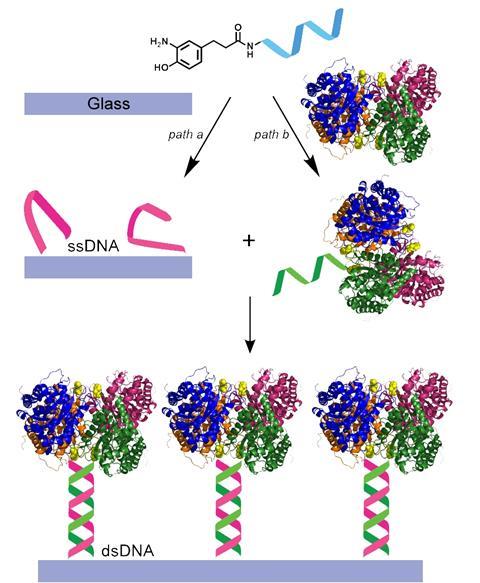



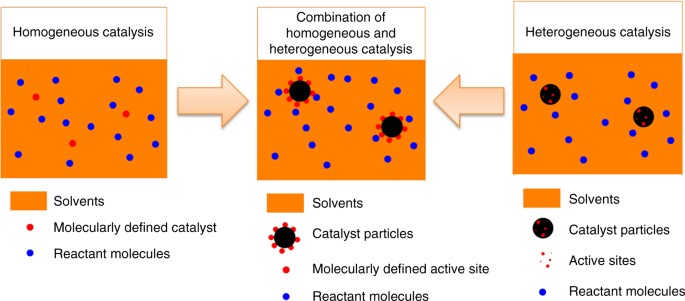
Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World




4 Examples O R Common Types O R Heterogeneous Catalysts Their Download Table



Heterogeneous Catalysis And Catalyst Recycling All About Drugs
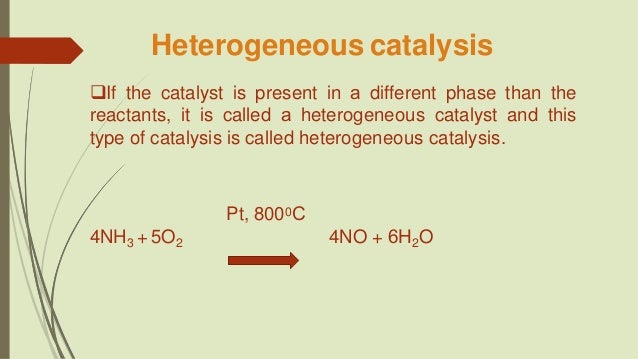



Heterogenous Catalysis Chemistry Is Love




Heterogeneous Catalysis All About Drugs



Catalysis Meaning Of Catalyst Its Characteristics And Types




Which Of The Following Reactions Is An Examples Of Homogeneous Catalysis Youtube



An Overview Of Different Types Of Catalysts Legal Advantage



Spatial And Temporal Exploration Of Heterogeneous Catalysts With Synchrotron Radiation Nature Reviews Materials




Single Atom Catalysis Bridging The Homo And Heterogeneous Catalysis Sciencedirect



Catalysis Ppt Video Online Download



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau




Catalysis Boundless Chemistry



Heterogeneous Catalysis All About Drugs



Types Of Catalysis



Catalysis In Industry




Chapter 13 8 Catalysis Chemistry Libretexts




Catalysts Transition Metals Secondary Science 4 All



Catalysis In Industry



Heterogeneous And Homogeneous Catalysis For The Hydrogenation Of Carboxylic Acid Derivatives History Advances And Future Directions Chemical Society Reviews Rsc Publishing
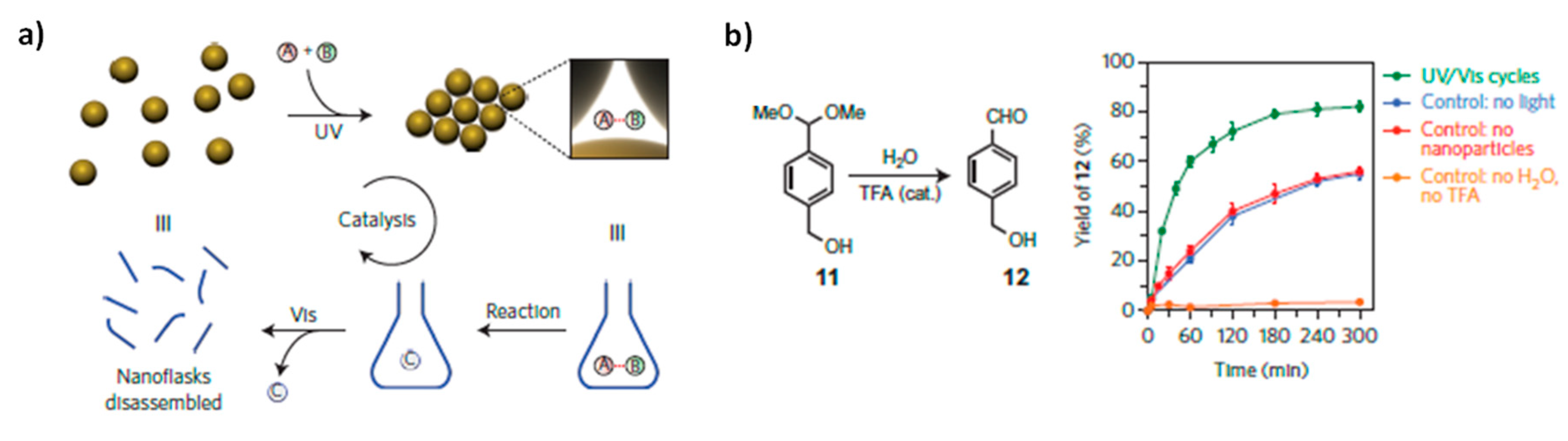



Catalysts Free Full Text Switchable Stimuli Responsive Heterogeneous Catalysis Html




Catalyst Ppt Video Online Download
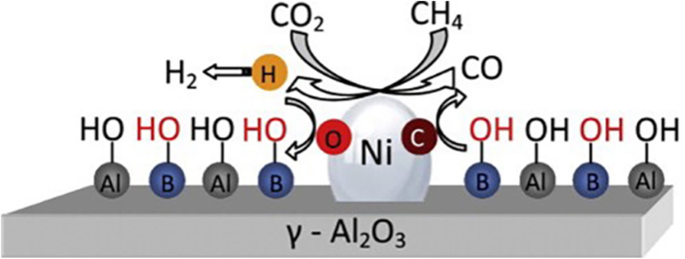



Heterogeneous Catalysts For Catalytic Co2 Conversion Into Value Added Chemicals Bmc Chemical Engineering Full Text
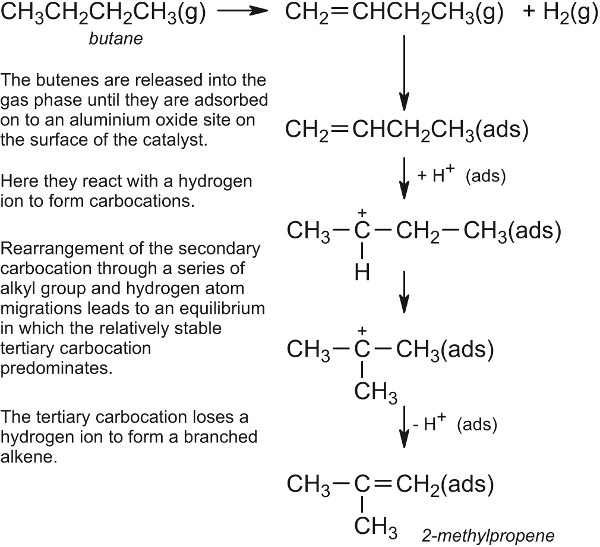



Catalysis In Industry




Adsorption Theory Of Heterogeneous Catalyst Definition Examples



Heterogeneous Catalysis All About Drugs
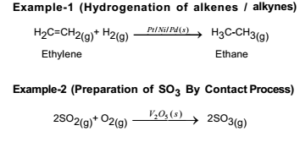



Catalysis Meaning Of Catalyst Its Characteristics And Types



Ppt Catalysts Powerpoint Presentation Free Download Id



Heterogeneous Catalysis Ppt Video Online Download



Q Tbn And9gcszztkjbzfsntexsurtvk4nl6npftcvaalxtfswqxoyuadjnltd Usqp Cau




Catalysis Boundless Chemistry



Catalysts Free Full Text Heterogeneous Catalysis On Metal Oxides Html




Heterogeneous Catalyst An Overview Sciencedirect Topics




Which Of The Following Reations Are Examples For Heterogeneous Catalysis Youtube



Ppt 23 5 Features Of Homogeneous Catalysis Powerpoint Presentation Free Download Id
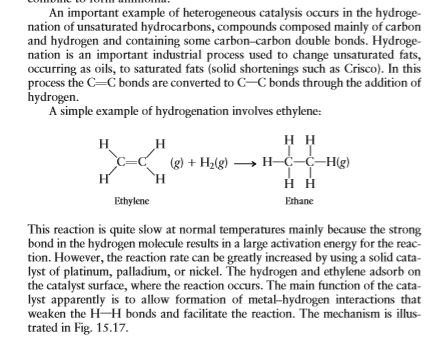



An Important Example Of Heterogeneous Catalysis Chegg Com




Give Four Examples Of Heterogeneous Catalysis




Pdf Heterogeneous Catalytic Chemistry By Example Of Industrial Applications Semantic Scholar
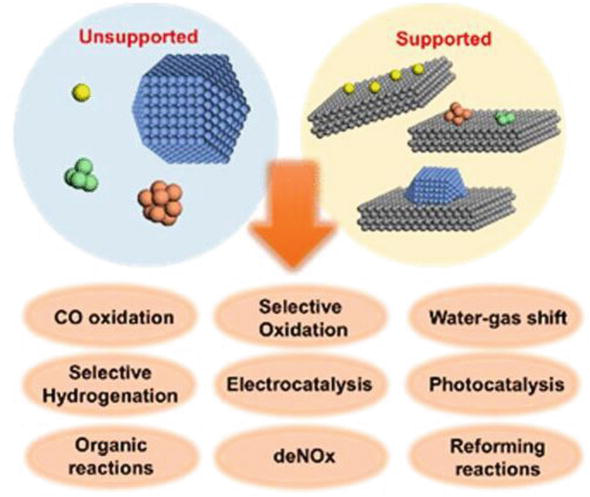



Heterogeneous Catalytic Process For Wastewater Treatment Intechopen




What Is Heterogeneous Catalysis Give An Example



How Do Catalysts Work



0 件のコメント:
コメントを投稿